
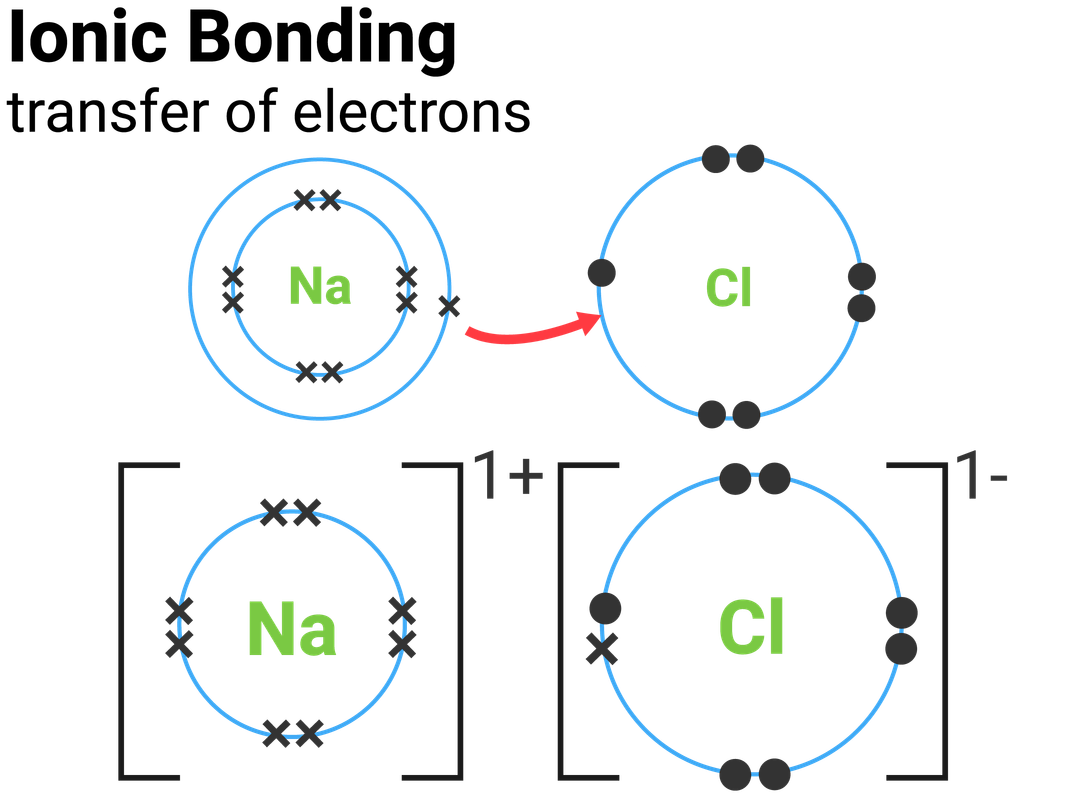
Because some atoms will lose electrons and some atoms will gain electrons, there is no overall change in the number of electrons, but with the transfer of electrons the individual atoms acquire a nonzero electric charge. One way is the transfer of electrons between two atoms until both atoms have octets. There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell.
This useful rule of thumb is called the octet rule, and it is a key to understanding why compounds form.
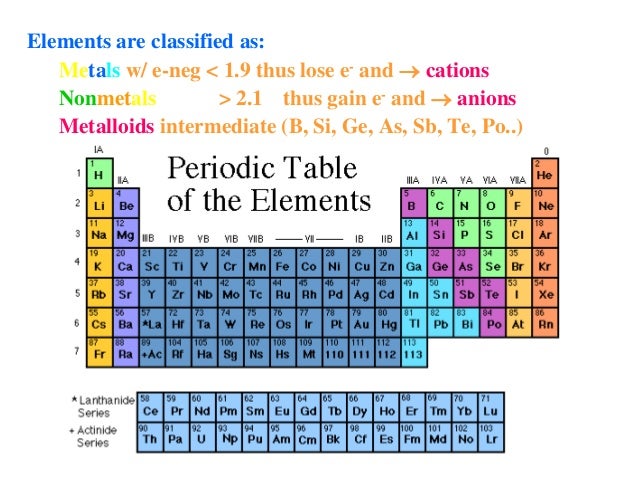
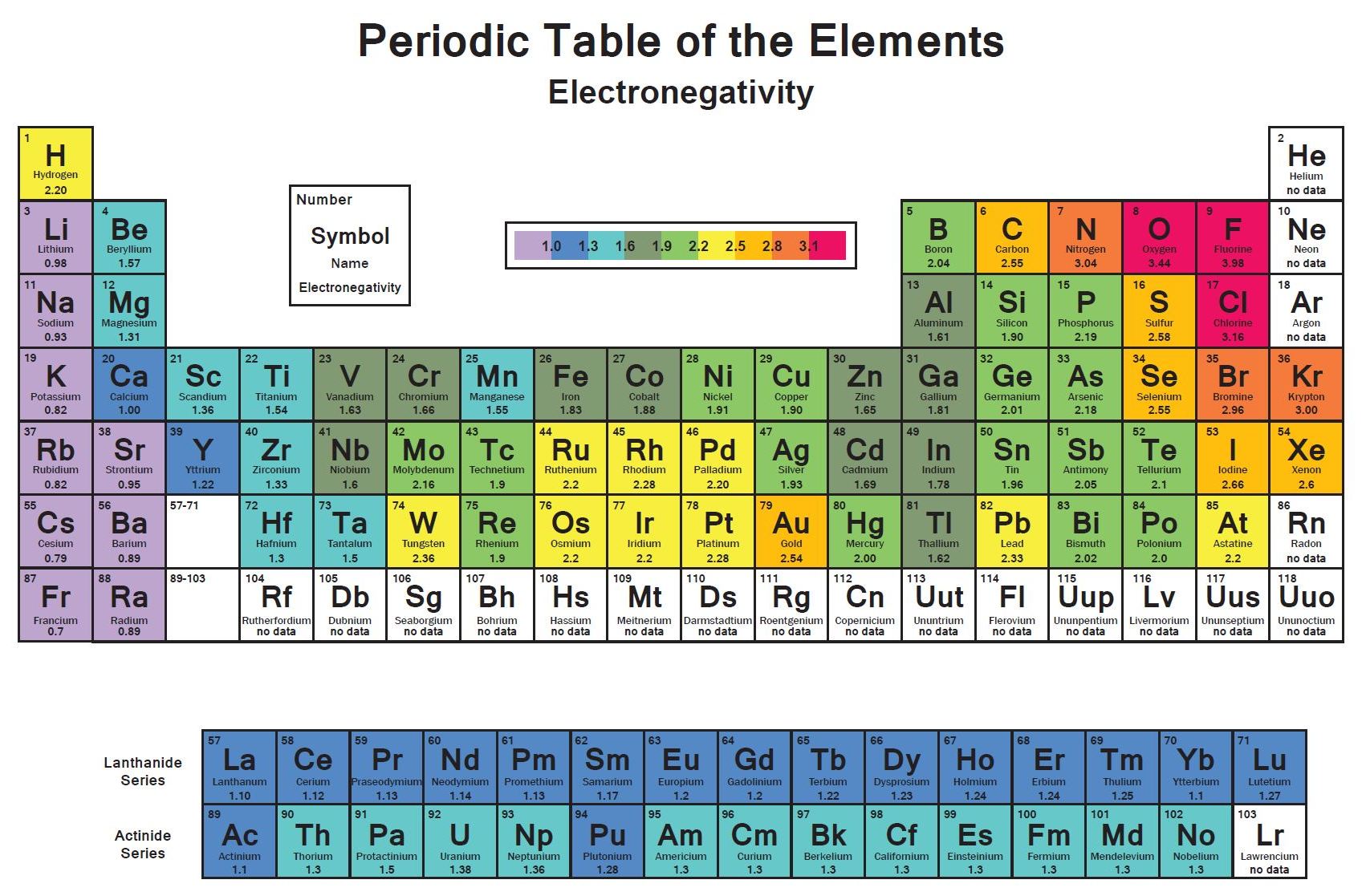
Chemists have concluded that atoms are especially stable if they have eight electrons in their outermost shell. What else do the noble gas elements have in common? Except for helium, they all have eight valence electrons. These elements-helium, neon, argon, krypton, xenon, and radon-do not form compounds very easily, which suggests that they are especially stable as lone atoms. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? A clue comes by considering the noble gas elements, the rightmost column of the periodic table. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart. 3.1 Two Types of BondingĪtoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. We will see additional examples of such differences in section 3.3 of this chapter covering “Covalent Bonding and Simple Molecular Compounds”, as we consider how atoms combine to form compounds. Nevertheless, the compound has properties completely different from either elemental sodium (a chemically reactive metal) or elemental chlorine (a poisonous, green gas). Table salt, as we have seen, consists of only two elements: sodium and chlorine. Compounds can be very complex combinations of atoms, but many important compounds are fairly simple. There are only 118 known chemical elements but tens of millions of known chemical compounds. 3 Covalent Bonding and Simple Molecular Compounds Covalent Bonds Electron Sharing Covalent Bonds Between Different Atoms Multiple Covalent Bonds 3.4 Chapter Summary Ionic Compounds Covalent Compounds 3. 2 Ions Electron Transfer Lewis Diagrams 3. This text is published under creative commons licensing, for referencing and adaptation, please click here.
Ion bonding table full#
For the interactive PDF, adobe reader is required for full functionality.
Ion bonding table pdf#
This content can also be downloaded as a PDF file.


 0 kommentar(er)
0 kommentar(er)
